In the current context of high-quality development in the pharmaceutical industry, water quality control has become crucial to drug quality and medication safety. Pharmaceutical water is not only an important raw material for drug production. But its purity is also a key factor affecting drug efficacy stability and reducing the risk of adverse reactions.
EDI water treatment equipment, as a new type of equipment to replace traditional ion exchange technology, is becoming the preferred solution for high-purity water preparation in the pharmaceutical industry due to its core advantages of no chemical pollution and stable water production.

How do EDI water treatment systems work?
EDI water treatment equipment is well-suited to the stringent requirements of the pharmaceutical industry due to its sophisticated technological logic. This equipment innovatively integrates ion exchange resins, selective ion exchange membranes, and a DC electric field to form a highly efficient water purification system.
Given the specific characteristics of pharmaceutical water, raw water must first undergo a precise pretreatment process to thoroughly remove suspended solids, colloids, microorganisms, and other contaminants.
It then enters the core EDI module. Inside the module, cation exchange membranes and anion exchange membranes are arranged alternately, creating independent desalination and concentrate discharge zones.
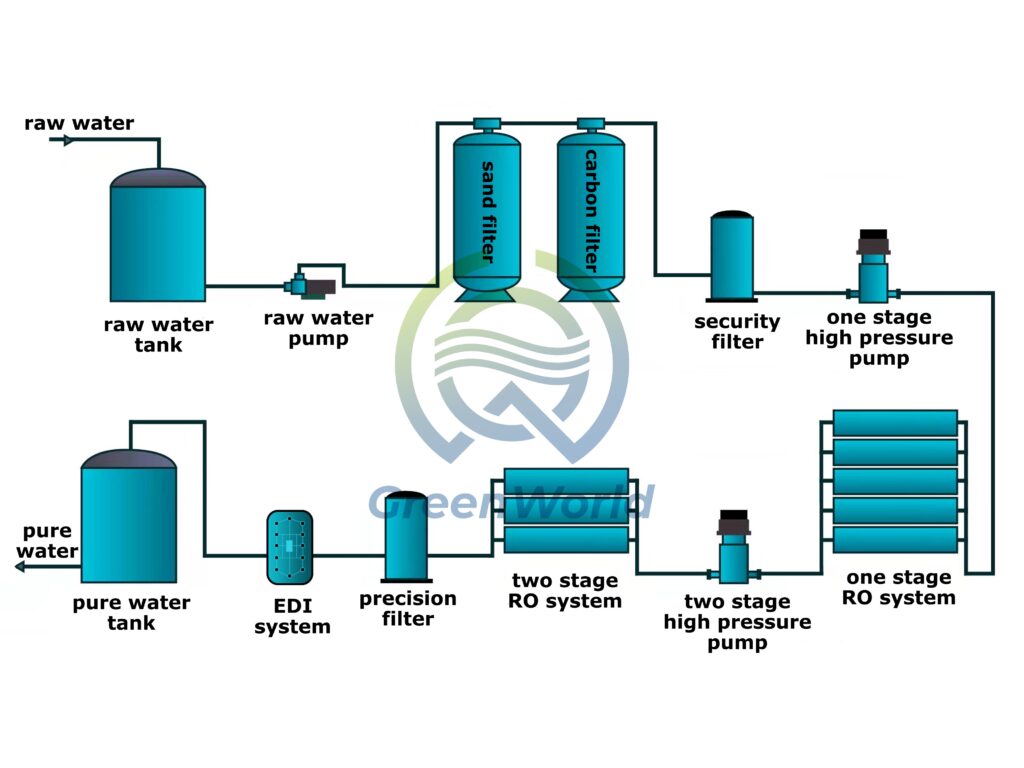
Driven by a direct current electric field, harmful ions in the water migrate directionally. For example, cations move towards the cathode, and anions move towards the anode, achieving efficient separation of ions and water molecules through the selective permeation of the membrane.
More importantly, the electric field continuously activates the regeneration capacity of the ion exchange resin, completely eliminating the reliance on acid-base regenerators in traditional processes and avoiding the risk of chemical pollution at the source.
Why does high-purity water preparation require EDI water treatment equipment?
Regarding water purity, the produced ultrapure water maintains a stable resistivity of 10-18 MΩ·cm, fully complying with the stringent standards for water for injection and sterile API production in the Good Manufacturing Practice (GMP) for pharmaceuticals. Simultaneously, it effectively avoids the adverse effects of impurity ions on drug synthesis reactions and formulation stability.
In terms of safety, the equipment requires no addition of acid or alkali reagents throughout its operation. Thus prevent the generation of chemical wastewater that could cause environmental pollution and eliminating the risk of cross-contamination due to regenerator residues.
At the operational level, although the initial equipment investment is relatively high, it eliminates subsequent costs such as regenerator procurement, wastewater treatment, and manual operation, reducing long-term operating costs by more than 30%. It also avoids the water production interruption problems during regeneration in traditional processes, ensuring the continuity of pharmaceutical production.
Which stages of the pharmaceutical industry use EDI water treatment equipment?
In the entire pharmaceutical industry chain, EDI (Electronic Dioxide) water treatment equipment has penetrated multiple key production stages.
① In injectable drug production, they use the sterile ultrapure water generated by our equipment for drug solution preparation and container cleaning—leveraging 15 years of industrial water treatment expertise to directly guarantee the sterility and stability of injectable drugs and prevent pyrogenic reactions caused by water quality issues.
② In the synthesis of sterile active pharmaceutical ingredients (APIs), high-purity water, as a reaction medium, can improve reaction efficiency and reduce impurity formation, ensuring that the purity of the API meets standards.

③ In the production of pharmaceutical excipients and drug packaging, they utilize EDI-generated water for excipient purification and precision cleaning of packaging materials
④ Furthermore, in pharmaceutical R&D laboratories, the stable high-purity water provided by EDI equipment offers reliable water quality support for experiments such as drug component analysis and dosage form development.
Summarize
In the future, with the innovation of ion exchange membranes and resin materials, EDI equipment will further enhance aseptic control capabilities while improving water production efficiency and reducing energy consumption. This will better meet the specific needs of high-end pharmaceutical fields such as biopharmaceuticals and gene-engineered drugs.











